|
Ion
Exchange
The
Short History
When
a salt is placed in aqueous solution,
it no longer exists as a salt but as charged particles
called ions. Sodium chloride (NaCl),
for instance, becomes Na+ (a cation)
and Cl- (an anion) and become associated
with the oppositely charged ions of the water itself:
H+ (cation) and OH- (anion). Mixed salts such as CaCl2
and NaNO3 dissociate not only into their respective ions
associated with the water ions but form associations with
ions from the other salt as well. Thus CaCl2 and NaNO3
exchange partners to form Na+ + Cl- and Ca++ + NO3- partnerships.
This is known as “ion exchange”.
However, since all four salts are highly soluble, separating
them into new “salt” components is very difficult
other than through complex steps of crystallization.
The ion exchange process has been around in nature since
the beginning of time although not observed and quantified
until the mid 1800s when sodium forms of naturally occurring
zeolites were shown to convert (exchange)
ammonium salts in solution to their sodium specie after
passing through a bed of the zeolite (which retained the
ammonium ions). This process was also shown to be reversible
by passing a strong solution of sodium salt through the
zeolite, thus stripping the ammonium ion and restoring
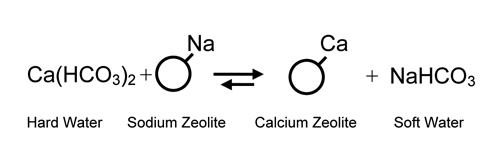
the bed to its sodium form. It was also demonstrated that
“hard” water containing calcium
(Ca++) and magnesium (Mg++) ions could be made “soft”
by exchanging the hard water ions for sodium. This gave
birth to the “softening”
process which was put into commercial application around
the turn of the 20th century. Regeneration
is with common salt.
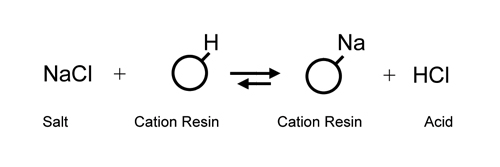
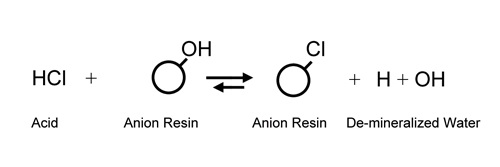
Utilizing
a cation exchanger to replace all cations in solution
with hydrogen ion (H+) and an anion exchanger
to replace all anions with hydroxide
(OH-) was not realized until the development of the modern
styrene/di-vinyl benzene resins in the
mid 1940s. It was now possible to substitute H+ and OH-
ions (H+ + OH- = water) for salt ions and effectively
de-mineralize water which had only been
possible through multi-step distillation before.
De-mineralization
via ion exchange has become the work horse of the high
purity water industry. Regeneration is with strong acid
and caustic respectively.
|
